LST Heavy Liquid for density separations
General Information on heavy liquids
Heavy liquids are dense fluids or solutions used to separate materials of different density through their buoyancy. Materials with a density greater than the heavy liquid will sink, while materials with a density less than the heavy liquid will float on the liquid surface.
In the mineral industry, heavy liquids are commonly used in the laboratory to separate the “light” minerals such as quartz and clay from the “heavy” minerals. The density used for this type of separation is about 2.85 g/ml, nearly three times the density of water. Heavy liquid separations are carried out for varied reasons, depending on the industry. The mineral sands industry uses heavy liquid separations to check the grade of samples in their process, and to determine the efficiency of their industrial hydrocyclone separations. The diamond exploration industry uses heavy liquids to separate the dense indicator minerals from sand and clay.
Another use of heavy liquids is in paleontology. Typically, these heavy liquid separations are conducted at a lower density (e.g. 2.2 g/ml) since the separation is not between minerals of different types, but between fossil bones and minerals.
Organic heavy liquids
The organic heavy liquids form the "older" generation of heavy liquids. Two of the organic heavy liquids most commonly in use are bromoform and tetrabromoethane (TBE). Of these, bromoform has the lower viscosity (1.8 cP) but is considered more hazardous to work with because it has the higher vapour pressure (5.9 mm Hg at 25°C). TBE has a higher viscosity (9 cP) and a lower vapour pressure (0.02 mm Hg at 25°C).
Another organic heavy liquid, used when higher densities are required, is methylene iodide. Methylene iodide has a density of 3.31 g/ml, a vapour pressure of 1.2 mm Hg at 25°C, and a low viscosity of 2.6 cP.
The three commonly used organic heavy liquids all have problems with toxicity, and must be handled in a fume hood. Methylene iodide is moderately toxic by subcutaneous and other routes. Bromoform has been shown to cause severe liver damage and is poisonous by ingestion. There is evidence that it is a human mutagen and a neoplastigen on experimental animals, so it is potentially carcinogenic. TBE is poisonous by inhalation or ingestion and is a moderate irritant to the skin. It is a neoplastigen and mutagen with animals.
These liquids have the problem of being volatile and potentially causing health problems. For this reason, they are being phased out in the USA.
Tungsten based heavy liquids: SPT, LMT and LST
Two low-toxicity substitutes for the organic heavy liquids bromoform and tetrabromoethane (TBE) have been available for some time. They are SPT (sodium polytungstate, or sodium metatungstate), and the lithium equivalent LMT which is lithium metatungstate. These are all inorganic compounds, based on the [H2W12O40]6- polyanion, which is dissolved in water to form very dense solutions.
In our experience, SPT (sodium polytungstate) and LMT (lithium metatungstate) are not used by many mineral labs which need a heavy liquid with a density of 2.8 g/ml or more, because their solutions at these densities are too viscous. From our testing, they are up to 5 times as viscous as LST Heavy Liquid. Typically the viscosity of these liquids is greater than 25 cP, or more than 25 times the viscosity of water. The higher viscosity of heavy liquids prepared from sodium metatungstate (also known as sodium polytungstate, or SPT) can be seen in the graph below. In practice, the higher viscosity of SPT leads to significantly slower and less efficient separations, as well as slow filtration.
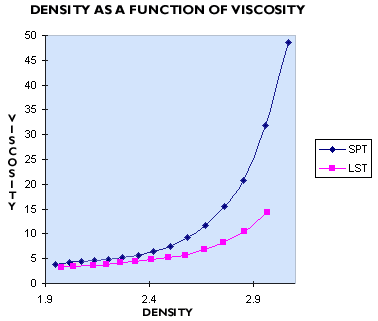
Another disadvantage of SPT and LMT is their lack of thermal stability above 80°C. The lack of thermal stability of sodium metatungstate and lithium metatungstate means that solutions of SPT or LMT cannot be evaporated by boiling, so the recirculation and regeneration of these liquids requires special equipment, or is quite time consuming.
LST Heavy Liquid has a low viscosity and excellent thermal stability. LST Heavy Liquid can be used up to a density of 2.9 g/ml at room temperature, and up to a density of 3.6 g/ml at elevated temperatures.

